Why Is Graphite Not the Best Conductor of Electricity
Graphite can conduct electricity because of the delocalised free electrons in its structure. In a graphite molecule one valence electron of each carbon atom remains free Thus making graphite a good conductor of electricity.
Why Is Graphite Not The Best Conductor Quora
But if your talking about pencil lead certainly silver is the better conductor.

. Metals conduct electricity as they have free electrons that act as charge carriers. The density of this element is around 22 gmcm3. Hence there wont be flow of electrons That is the reason.
Bonding the three carbon atoms to each carbon atom is covalent. This means that while graphite might not be the best conductor as there is only one delocalised electron per carbon atom it is still a good conductor of electricity unlike another allotrope of carbon diamond which. It is because of free electron in graphite.
Why graphite is a poor conductor of electricity at high temperature. The very reason why metals do. Thus this makes graphite a poor conductor.
Hence there wont be flow of electrons That is the reason behind diamond are bad conductor electricity. Whereas in diamond they have no free mobile electron. Why is graphite a good conductor of electricity and diamond is not.
But diamond do not contain any free electon and all electrons are covalently bonded. Graphite is just the same says Dr Dong Liu physics lecturer at the University of Bristol. In order to conduct electricity graphite must be flexible.
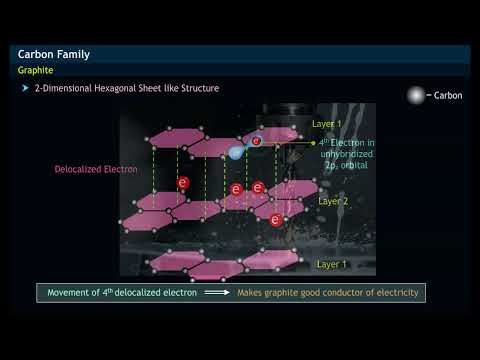
Diamond on the other hand consists of a 3D lattice structure with each carbon atom bonded to four. These free electrons make graphite a good conductor of electricity. There is a great deal of distance between planes and they are bonded weakly together allowing the.
In a graphite molecule one valence electron of each carbon atom remains free Thus making graphite a good conductor of electricity. These arise because each carbon atom is only bonded to 3 other carbon atoms. Graphite is a good conductor of electricity because its electrons are delocalized or free to move around.
While the fourth carbon remains free between the sheets and conducts electricity the electrons of carbon atom 3 are released from the fourth sheet to form a bond. Whereas in diamond they have no free mobile electron. Graphite can conduct electricity because of the delocalised free electrons in its structure.
Due to its delocalised electrons graphite provides a conductor for heat and electricity. Copper is a better conductor than graphite for electricity due to its lattice structure with free electrons that can transmit energy across the structure whilst graphite is still able to conduct electricity it is not as strong a conductor as copper. Graphite is a good conductor of electricity as each carbon atom is linked to three of its neighbouring carbon atoms.
Why is graphite a good conductor of electricity Class 10. Graphite is a good conductor of electricity because of the presence of free electrons in its crystal. Gold is a good conductor of both electricity and heat.
As she points out graphite is made from carbon atoms which have four electrons in their outer shells. Because of perfect crystalline structure of diamond is hard and non-availability of free electrons it is a bad conductor of electricity. I would say that graphite has a 3D network.
Hence there wont be flow of electrons That is the reason. At higher temperature graphite has an intermolecular structure with immobile ions. Soils are electric current conductors.
Thus graphite does conduct electricity as it contains delocalised electrons that carry charge and are free to move throughout the graphite lattice. Why does graphite conducts electricity but not Diamond Class 10. Graphite consists of several 2D layers of covalently bonded atoms stacked together.
Answer 1 of 2. Hence there wont be flow of electrons That is the reason. Gold is a chemical element with the atomic number 79.
Their configuration in this substance allows electrons to flow freely and thus conduct electricity which is merely the flow of electrons from one place to another. However in diamond all 4 outer electrons on each carbon atom are used in covalent bonding so there are no delocalised electrons. Whereas in diamond they have no free mobile electron.
Graphite is insoluble in water or any other solvents because of the strong bond within the graphite element however soluble in warm chlorosulfuric acid and molten nickel. I think the easy answer would be silver. This means that the fourth valence electron of each carbon atom is free.
Why does graphite conduct electricity whereas diamond does not. In a graphite molecule one valence electron of each carbon atom remains free Thus making graphite a good conductor of electricity. In a graphite molecule one valence electron of each carbon atom remains free.
Due to the free electrons in its framework graphite can perform electricityTherefore graphite is said to be a good conductor of electricity. Presence of free electrons in graphite makes graphite a good conductor of electricity and it acts as soft lubricant because of layered structure. Graphite is a crystalline form of carbon with its atoms arranged in a hexagonal structure.
It has the properties of bot metals and non-metals. In a graphite molecule one valence electron of each carbon atom remains free Thus making graphite a good conductor of electricity. Graphite is a good conductor of electricity.
Graphite is a very good conductor of electricity because of the presence of the delocalized electron. Graphite is structured into planes with tightly bound atoms. Whereas in diamond they have no free mobile electron.
Only carbon atoms make up the whole structure. Copper is a better conductor than graphite for electricity due to its lattice structure with free electrons that can transmit energy across the structure whilst graphite is still able to conduct electricity it is not as strong a conductor as copper. Thats why diamond are bad conductor electricity.
Whereas in diamond they have no free mobile electron. In contrast diamond has no free mobile electron so diamond is a bad conductor of electricity. Graphite is a good conductor of electricity since each carbon atom is linked to three of its neighbouring carbon atoms.
Graphite is an allotrope of carbon. But I suppose it could depend on the molecular structure and temperature of the graphite. As carbons are linked 3 other C atoms hexagonal sheets are produced from hexagonal elements.
So the fourth electron is free to move which can carry a charge. In a graphite molecule one valence electron of each carbon atom remains free Thus making graphite a good conductor of electricity.

Quick Answer Why Graphite Is A Good Conductor Of Electricity 2 Seniorcare2share

Graphite Is A Good Conductor Of Heat And Electricity Because It Contains
Does The Softness Of Graphite Make It Usable As A Conductor Of Heat A Conductor Of Electricity Or A Lubricant For An Electrode Quora

Why Diamond Is A Non Conductor While Graphite Is A Good Conductor Of Electricity Seniorcare2share

To Prove That Graphite Is A Good Conductor Of Electricity Science Experiment Youtube
Quick Answer Why Graphite Is A Good Conductor Of Electricity 2 Seniorcare2share
Aside From Metals What Else Are Good Conductors Quora
Is Inorganic Graphite A Good Conductor Of Electricity Quora

Diamond Is Insulator But Graphite Is A Conductor Why

Answer The Following Question Why Is Graphite A Good Conductor Of Electricity But Not Diamond

Why Is Graphite A Good Conductor Of Electricity Dancrabon

Answer The Following Question Why Is Graphite A Good Conductor Of Electricity But Not Diamond
Why Is Graphite Not The Best Conductor Quora

Although More Sophisticated Models For The Structure Of Metallic Solid Are Available Including The Band Model Most Properties Of Metals Can Be Explained By A Relatively Simple Model Based On The Fact

Bond Why Is Fullerene 60 An Insulator While Graphite Is A Conductor Chemistry Stack Exchange

Quick Answer Is Diamond A Good Or Bad Conductor Of Heat Shine Precious Stones
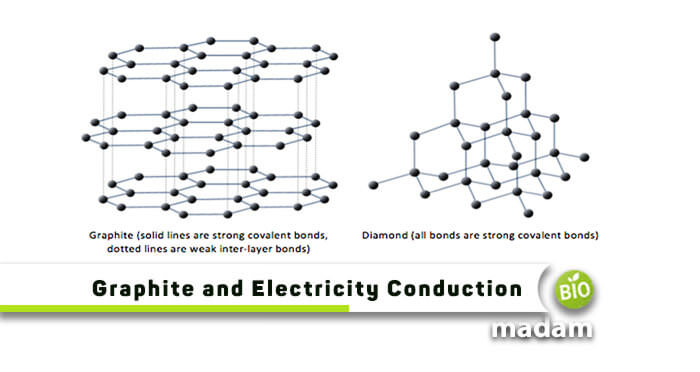
Is Graphite A Good Conductor Of Electricity Biomadam

Chemistry 1 Question Graphite Is Best Conductor Of Electricity But Isnt Used To Make Wires Why Science Coal And Petroleum 11558587 Meritnation Com
Comments
Post a Comment